歡迎關(guān)注「漢莎科技集團」微信公眾號!
近年來��,快速葉綠素?zé)晒庹T導(dǎo)動力學(xué)曲線(OJIP曲線)及其數(shù)據(jù)分析方法JIP-test在植物科學(xué)研究中的應(yīng)用越來越廣泛(BussottiF, et al., 2020; KalajiH M, et al., 2017; Pontes. D, 2019; Tsimilli-michael M, 2020)����。OJIP曲線可以更直觀地表現(xiàn)出差異�����,JIP-test則提供豐富的參數(shù)���,由于其測定方便簡單��,逐漸成為科研工作者們研究光合作用原初光化學(xué)反應(yīng)的有力工具����。
在植物科學(xué)實驗中,測定的實驗數(shù)據(jù)非常多���,比如光合作用參數(shù)����、植物生長指標(biāo)��、各種酶活性以及分子實驗數(shù)據(jù)等���,再加上JIP-test本身提供的幾十種參數(shù)�����,豐富實驗數(shù)據(jù)的同時�����,也會給后期的處理帶來很大的工作量����。因此,采用準(zhǔn)確的數(shù)據(jù)處理分析方法尤其重要����。主成分分析法(PCA)是數(shù)據(jù)挖掘中常用的一種降維算法。所謂降維���,就是把具有相關(guān)性的變量數(shù)目減少����,用較少的變量來取代原先變量���。在植物科學(xué)研究的實際應(yīng)用中,各種參數(shù)相互之間會有影響���,通過PCA分析處理后��,會得到有限的幾個主成分�����,由其代表實驗參數(shù)就可以說明實驗問題了����,也就是所謂的降維(KalajiH M, et al., 2018;Goltsev V, et al., 2012)。
JIP-test提供豐富的參數(shù)��,PCA進(jìn)行數(shù)據(jù)降維處理����,兩者結(jié)合,能夠快速處理并分析大量的實驗數(shù)據(jù)�,(i)揭示影響實驗的主要參數(shù),并可(ii)聚類不同處理之間的差異����,也可用于(iii)大數(shù)據(jù)分析并預(yù)測植物生長變化。下面通過三篇文章來詳細(xì)介紹二者的結(jié)合應(yīng)用�。
1. 解析參數(shù)間的相關(guān)性,篩選出可禁用詞匯解釋問題的參數(shù)(Jurczyk B�����,2015)
近年來�,全球范圍內(nèi)短期內(nèi)澇等自然災(zāi)害頻發(fā),并且隨著北半球高緯度地區(qū)秋冬季降水量的增加��,這種情況的出現(xiàn)可能會更加頻繁。研究結(jié)果表明����,淹水溫度是影響植物對該脅迫反應(yīng)的重要因素。該試驗研究了耐寒性不同的四種高羊茅在低溫下對土壤水分過剩的光合機構(gòu)響應(yīng)�����,旨在驗證Rubisco活性改變引起的葉片水溶性碳水化合物濃度變化是否會影響土壤水分過剩條件下的光適應(yīng)�。
通過研究低溫淹水對葉綠素 a 熒光參數(shù)、水溶性碳水化合物(WSC)����、Rubisco活化酶基因表達(dá)(RcaA)和Rubisco活性(RA)的影響,并進(jìn)行PCA主成分分析�����,以減少需要進(jìn)行詳細(xì)分析的參數(shù)數(shù)量�����,并篩選出能禁用詞匯解釋問題的參數(shù)�。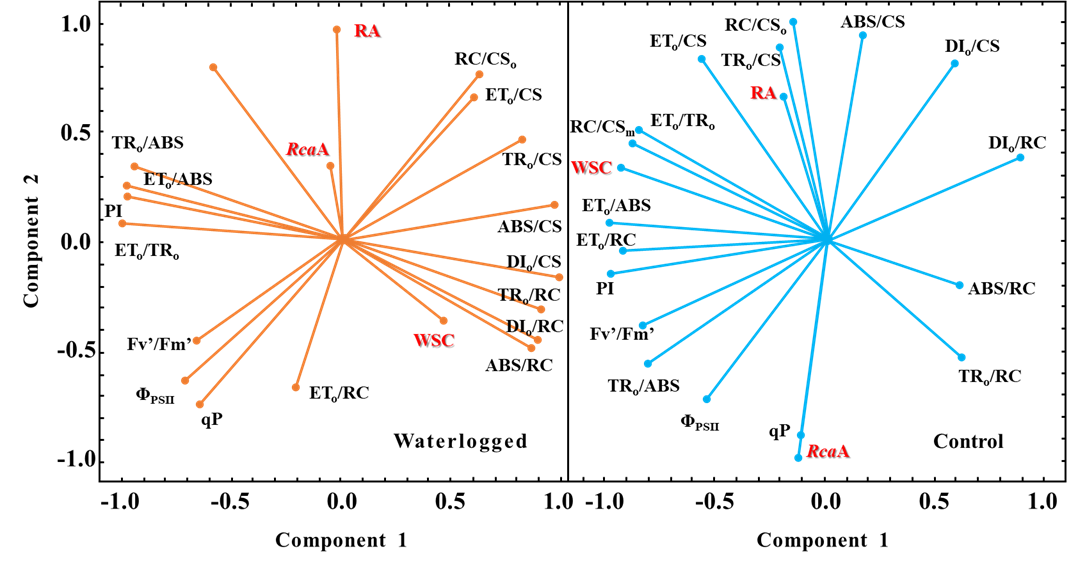
圖1. 主成分分析的向量圖���,顯示了低溫對照和低溫淹水被調(diào)查變量之間的相關(guān)性
淹水脅迫會直接導(dǎo)致植物水下組織供氧不足����,缺氧后植物會加速使用碳儲備進(jìn)而導(dǎo)致碳源供應(yīng)不足。主成分分析證實����,由圖1可以看出,淹水脅迫后�,被測變量之間的關(guān)系發(fā)生顯著性改變。在對照條件時����,水溶性碳水化合物與能量傳遞效率相關(guān)參數(shù)(ETo/TRo、ETo/ABS�����、ETo/RC)有很高的正相關(guān)性�����,說明WSC的積累在對照條件下是不受限制的���;淹水后�,WSC與能量耗散效率(DIo/CS、DIo/RC)呈正相關(guān)����,說明能量轉(zhuǎn)移的干擾可能限制了WSC的濃度。另一方面����,WSC與描述單個活性反應(yīng)中心效率的參數(shù)高度相關(guān),揭示了類囊體膜可能也因淹水受到損傷��。此外�����,qP和RcaA在對照植株中的表達(dá)之間的強相關(guān)性可能表明這兩個性狀的調(diào)控機制相似���,可能與ADP/ATP比值有關(guān)����。在淹水條件下��,qP和RcaA的表達(dá)不相關(guān)�,提示另一個因素可能調(diào)節(jié)RcaA轉(zhuǎn)錄水平。總的來說低溫淹水后���,酶活性劇烈下降�,光反應(yīng)階段吸收的光能過剩���,維持較高的WSC含量能夠激活光合作用適應(yīng)寒冷的熱耗散機制��,有助于耗散掉過剩光能��。2. 聚類分析不同處理之間的差異(Zhiponova M, 2020)
光是控制植物生長發(fā)育的主要因素����。光不僅推動植物光合作用���,光質(zhì)和光照時間還驅(qū)動著植物主要的發(fā)育變化�,如光形態(tài)發(fā)生�、開花的光周期誘導(dǎo)、向光性����、避蔭以及防御等。為了評估光照條件對植物生理狀態(tài)的影響�����,該研究在豌豆植株的早期發(fā)育過程中使用正常白光(W)、白色陰影(WS)�、高光強藍(lán)/紅/遠(yuǎn)紅組合光(BR)和低光強藍(lán)/紅/遠(yuǎn)紅組合光(BRS)四種光照射,采用JIP-test來評估與光吸收和電子傳輸有關(guān)的PSII參數(shù)����,并通過PCA技術(shù)聚類分析不同光照之間的差異。
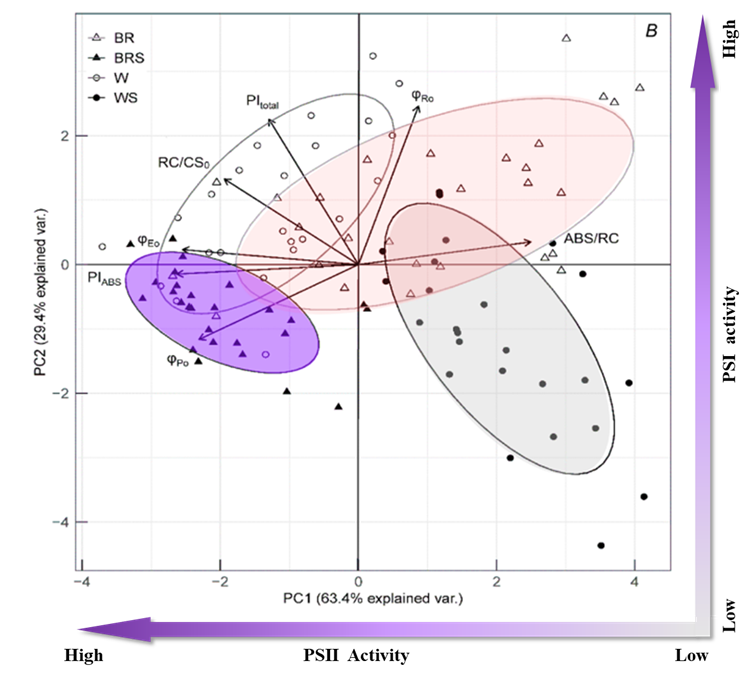
圖2. JIP-test參數(shù)和不同處理的主成分分析(Plant variants: W – white light; WS – white light with shadow; BR – blue and red light; BRS – blue and red light with shadow)
對獲得的JIP-test參數(shù)進(jìn)行主成分分析表明�,盡管不同處理之間存在重疊,但它們對光合機構(gòu)的影響差異還是很容易區(qū)分的����。PC1根據(jù)PSII活性分離出不同的處理,較低的值表示更高的PSII性能(低光吸收���、高光化學(xué)和電子傳輸效率)��;PC2則對應(yīng)PSI活性���,較高的值表明PSI性能較高。W處理表現(xiàn)出PSI和PSII的禁用詞匯綜合性能�����;WS處理表現(xiàn)出PSI和PSII的禁用詞匯綜合性能����;BR處理表現(xiàn)出受損的PSII和完整的PSI活性�;BRS處理表現(xiàn)出低PSI和完整的PSII性能���。結(jié)合其他生理數(shù)據(jù)和主成分分析可以揭示光合作用與開花的關(guān)系。具有高PSII表現(xiàn)(-PC1)的W和BRS處理在其發(fā)育后期發(fā)育出相同數(shù)量的花�,而具有抑制PSII活性(+PC1)的WS和BR植株發(fā)育后期沒有開花。研究結(jié)果表明���,PIABS在PC1上最相關(guān)���,可作為預(yù)測豌豆開花數(shù)量的最準(zhǔn)確指標(biāo)。3. 通過PCA技術(shù)對大樣本試驗進(jìn)行大數(shù)據(jù)分析(Bussotti F, 2020)
在大規(guī)模生態(tài)調(diào)查中�����,為了達(dá)成篩選目的和效率���,一般使用有限的參數(shù)來對樣本進(jìn)行快速��、簡單的評價和生理分類���。人們提出了許多形態(tài)���、化學(xué)和生理指標(biāo)來評價生態(tài)系統(tǒng)中的植物狀況。其中�,葉綠素的瞬時熒光分析(JIP-test)被認(rèn)為特別適用于大型生態(tài)調(diào)查,并能在短時間內(nèi)篩選出許多樣品�����。
JIP-test提供了五十多個參數(shù)來評估植物光合機構(gòu)的光化學(xué)性質(zhì)和功能�,這些參數(shù)可以描述光化學(xué)過程在能量吸收、俘獲和電子傳輸方面的不同階段����。該研究采用主成分分析法(PCA)對過去在野外條件(森林、人工林和牧場)和實驗室條件中獲得的43987個測量數(shù)據(jù)進(jìn)行分析��,目的是探討JIP-test參數(shù)之間的關(guān)系����,以選擇最合適的參數(shù)來捕捉植物光合效率的變異性及其對逆境的響應(yīng)。
本研究中分析的最大數(shù)據(jù)集源自FunDivEUROPE項目(Functional Significance ofthe Forest Diversity in Europe, European Union, 7th FrameworkProgram)��。此項目中分析了整個歐洲的森林生態(tài)系統(tǒng)����,從地中海到歐洲北部地區(qū)��,涵蓋了豐富的差異樹種組成�����。其中包括天然高大森林(In Italy, Spain, Romania,Poland,Finland, Baeten et al., 2013)和人工林場(In Finland and Germany, seeVerheyen et al.,2016)��。
通過PCA技術(shù)分析發(fā)現(xiàn),所選的JIP-test參數(shù)形成了三個很好分離的簇�。其中兩個位于PC1(Cluster 1&2)上,一個(Cluster 3)位于PC2上�����。每一組參數(shù)描述了不同的生理過程:光能捕獲和光化學(xué)階段(Cluster 1)����、耗散(Cluster 2)和熱階段(Cluster 3)。基于PCA分析��,該研究認(rèn)為樣品的整體光合性能可以用PITOT來表示����,或者用Fv/Fm和ΔVIP共同來表示。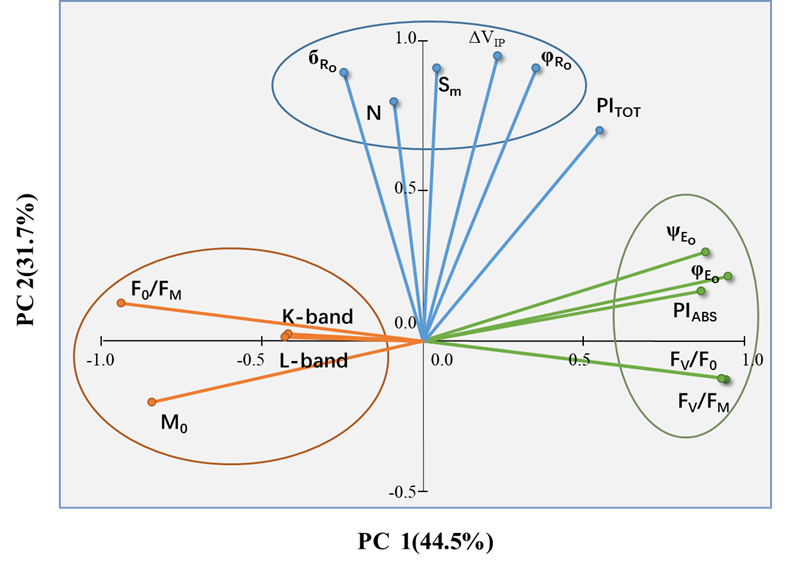
圖3. 所選JIP-test參數(shù)的主成分分析
在大多數(shù)情況下�,植物的光合性能可用Fv/Fm和ΔVIP來描述。經(jīng)過驗證�����,F(xiàn)v/Fm和ΔVIP能夠有效地代表各種調(diào)查(野外和實驗室)�、氣候和時間跨度、植物物種和功能群(針葉樹和闊葉樹物種����、草本植物)中樣本的變異性。因此�����,可用于探索性調(diào)查���,以篩選大樣本植物的光合性能���,以及它們對不同生態(tài)條件的適應(yīng)性。在林業(yè)或生態(tài)學(xué)調(diào)查方面���,以JIP-test為代表的植被葉綠素?zé)晒馓匦缘拇笠?guī)模野外調(diào)查對驗證無人機或衛(wèi)星遙感觀測結(jié)果具有重要意義����,遙感觀測數(shù)據(jù)和野外實地調(diào)查數(shù)據(jù)之間的銜接將是今后生態(tài)學(xué)研究的一個重要領(lǐng)域(Bussotti F, 2020)!以上實例說明���,PCA分析與JIP-test結(jié)合應(yīng)用越來越廣泛�����,大大提高了數(shù)據(jù)分析效率�����,能夠快速判斷實驗處理后的主要變化,并分析主要影響因素���,從而對實驗材料進(jìn)行預(yù)判����。近年來��,PCA在植物科學(xué)研究中的應(yīng)用呈上升趨勢�,相信科研工作者們會開發(fā)出更多更好的應(yīng)用方向。
如何實現(xiàn)對葉綠素a熒光數(shù)據(jù)(JIP-test參數(shù))、其它生理參數(shù)和基因��、蛋白等分子數(shù)據(jù)組成的大數(shù)據(jù)庫進(jìn)行PCA分析�?
通常我們可以使用學(xué)術(shù)界常用的商用數(shù)據(jù)分析軟件進(jìn)行PCA分析,如SPSS Statistics(IBM Corp)�、Statistica(StatSoft Inc. 2011)和SAS(SAS Enterprise Miner; SAS Institute, Cary, NC)等。
在全球互聯(lián)網(wǎng)化的大趨勢下�����,也涌現(xiàn)出一批使用體驗更佳�����、分析更加智能化的在線數(shù)據(jù)分析工具��,如SPSSAU(QingSi Technology Ltd 2016-2020)�、ClustVis(Metsalu, Tauno et al. 2015)等。
此外以R語言和Python為代表的計算機程序設(shè)計語言可以實現(xiàn)對大數(shù)據(jù)的快速智能處理��、計算和制圖���,使用R語言和Python對JIP-test熒光數(shù)據(jù)進(jìn)行PCA數(shù)據(jù)分析也已有非常成熟的語言包進(jìn)行應(yīng)用���。
下期文章我們將以IBM SPSS Statistics 26為例詳細(xì)介紹JIP-test熒光參數(shù)PCA分析操作方法����,敬請期待���!

本文參考文獻(xiàn)
[1] Baeten,L., Verheyen, K., et al. A novel comparative research platform designed to determinethe functional significance of tree species diversity in European forests. Perspect.Plant Ecol. Evol. Syst. 2013, 15 (5), 281–291.
[2] Bussotti F, Gerosa G, Digrado A, et al.Selection of chlorophyll fluorescence parameters as indicators ofphotosynthetic efficiency in large scale plant ecological studies[J].Ecological Indicators, 2020, 108: 105686.
[3] Goltsev V, Zaharieva I, Chernev P, et al.Drought-induced modifications of photosynthetic electron transport in intactleaves: analysis and use of neural networks as a tool for a rapid non-invasiveestimation[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2012,1817(8): 1490-1498.
[4] Kalaji H M, Bąba W, Gediga K, et al.Chlorophyll fluorescence as a tool for nutrient status identification inrapeseed plants[J]. Photosynthesis research, 2018, 136(3): 329-343.
[5] Kalaji H M, Schansker G, Brestic M, et al.Frequently asked questions about chlorophyll fluorescence, the sequel[J].Photosynthesis Research, 2017, 132(1): 13-66.
[6] Metsalu, Tauno and Vilo, Jaak. Clustvis: a webtool for visualizing clustering of multivariate data using Principal ComponentAnalysis and heatmap. Nucleic Acids Research, 43(W1): W566–W570, 2015.
[7] Pontes. D, Ontes, M.,Rodriguez, R. and Santiago,E.F. Letter to The Editor. The energy flux theorycelebrates 40 years: toward asystems biology concept? Photosynthetica,2019, vol. 57, iss. 2, p.521-522.
[8] Tsimilli-michael M. Revisiting JIP-test: Aneducative review on concepts, assumptions, approximations, definitions andterminology[J].Photosynthetica,2019,57 (SI): 90-107.
[9] Verheyen, K., Vanhellemont, M., et al. 2016.Contributions of a global network of tree diversity experiments to sustainableforest plantations. Ambio 45, 29–41.
應(yīng)用PCA分析JIP-test熒光數(shù)據(jù)文獻(xiàn)節(jié)選
[1] DimitrovaS, Paunov M, Pavlova B, et al. Special issue in honour of Prof. Reto J.Strasser–Photosynthetic efficiency of two Platanus orientalis L. ecotypesexposed to moderately high temperature-JIP-test analysis[J]. Photosynthetica,2020, 58(SPECIAL ISSUE): 657-670.
[2] BussottiF, Gerosa G, Digrado A, et al. Selection of chlorophyll fluorescence parametersas indicators of photosynthetic efficiency in large scale plant ecologicalstudies[J]. Ecological Indicators, 2020, 108: 105686.
[3] MihaljevićI, Lepeduš H, Šimić D, et al. Photochemical efficiency of photosystem II in twoapple cultivars affected by elevated temperature and excess light in vivo[J].South African Journal of Botany, 2020, 130: 316-326.
[4] Franić M,Jambrović A, Šimić D, et al. Photosynthetic properties of maize hybrids underdifferent environmental conditions probed by the chlorophyll a fluorescence[J].Maydica, 2020, 64(3): 9.
[5] Plich J,Boguszewska-Mańkowska D, Marczewski W. Relations Between PhotosyntheticParameters and Drought-Induced Tuber Yield Decrease in Katahdin-Derived PotatoCultivars[J]. Potato Research, 2020: 1-15.
[6] Vuletic M,Španic V. Special issue in honour of Prof. Reto J. Strasser–Characterization ofphotosynthetic performance during natural leaf senescence in winter wheat:Multivariate analysis as a tool for phenotypic characterization[J]. Photosynthetica2020, 58(2):301-313
[7] Faria-SilvaL, Gallon C Z, Silva D M. Photosynthetic performance is determined byscion/rootstock combination in mango seedling propagation[J]. ScientiaHorticulturae, 2020, 265: 109247.
[8] Banr A,Bruggemann W. Special issue in honour of Prof. Reto J. Strasser–Comparativeanalysis of drought stress response of maize genotypes using chlorophyllfluorescence measurements and leaf relative water content[J]. Photosynthetica,2020, 58(SPECIAL ISSUE): 638-645.
[9] CarreirasJ, Pérez-Romero J A, Mateos-Naranjo E, et al. The effect of heavy metalcontamination pre-conditioning in the heat stress tolerance of native andinvasive Mediterranean halophytes[J]. Ecological Indicators, 2020, 111: 106045.
[10] BlackhallV, Orioli G A, Colavita M G. JIP-test parameters to study apple peelphotosystem II behavior under high solar radiation stress during fruitdevelopment[J]. Photosynthetica 2020, 58(2):314-322
[11] SwoczynaT, Latocha P. Monitoring seasonal damage of photosynthetic apparatus in maturestreet trees exposed to road-side salinity caused by heavy traffic[J]. Photosynthetica58 (SI): 388-399, 2020
[12] Gast A,Romermann C, Bucher S F. Seasonal variation and trade-off between frostresistance and photosynthetic performance in woody species[J]. Photosynthetica2020, 58(2):331-340
[13] WiewióraB, Żurek G, Rybka K, et al. The origin of Epichloë endophyte-perennial ryegrasssymbionts modify plant reactions to elevated concentration of Pb2+, Cd2+ andCu2+ ions in soil[J]. BMC Plant Biology,2020.
[14] Samborska-SkutnikI A, Kalaji H M, Sieczko L, et al. Special issue in honour of Prof. Reto J.Strasser–Structural and functional response of photosynthetic apparatus ofradish plants to iron deficiency[J]. Photosynthetica 2020, 58(2):205-213
[15] Janka E,Umetani I, Sposob M, et al. Special issue in honour of Prof. Reto J.Strasser–Photosynthesis response of microalgae (Tetradesmus wisconsinensis) todifferent inorganic carbon sources probed with chlorophyll fluorescenceanalysis[J].Photosynthetica 2020, 58(2):236-244
[16] Galic V,MAZUR M, ŠIMIĆ D, et al. Special issue in honour of Prof. Reto J.Strasser–Plant biomass in salt-stressed young maize plants can be modelledwithphotosynthetic performance[J]. Photosynthetica 2020, 58(2):194-204
[17] ZhiponovaM, Paunov M, Anev S, et al. JIP-test as a tool for early diagnostics of plantgrowth and flowering upon selected light recipe[J]. Photosynthetica 58 (SI):399-408, 2020
[18] Arslan Ö,Nalcaiyi A S B, Erdal Ş Ç, et al. Analysis of drought response of sunflowerinbred lines by chlorophyll a fluorescence induction kinetics[J]. Photosynthetica2020, 58(2):348-357
[19] SwoczynaT, Łata B, Stasiak A, et al. JIP-test in assessing sensitivity to nitrogendeficiency in two cultivars of Actinidia arguta (Siebold et Zucc.) Planch. exMiq[J]. Photosynthetica, 2019, 57(2): 646-658.
[20] SamborskaI A, Kalaji H M, Sieczko L, et al. Can just one-second measurement ofchlorophyll a fluorescence be used to predict sulphur deficiency in radish(Raphanus sativus L. sativus) plants[J]. Current Plant Biology, 2019, 19:100096.
[21] Galić V,Mazur M, Šimić D, et al. Plant biomass in salt-stressed young maize plants canbe modelled with photosynthetic performance[J]. Photosynthetica, 2019, 57:9-19.
[22] Faria-SilvaL, Gallon C Z, Filgueiras P R, et al. Irrigation improves plant vitality inspecific stages of mango tree development according to photosyntheticefficiency[J]. Photosynthetica, 2019, 57(3): 820-829.
[23] PietriniF, Carnevale M, Beni C, et al. Effect of Different Copper Levels on Growth andMorpho-Physiological Parameters in Giant Reed (Arundo donax L.) inSemi-Hydroponic Mesocosm Experiment[J]. Water, 2019, 11(9): 1837.
[24] Xie Y,Sun X, Feng Q, et al. Comparative physiological and metabolomic analyses revealmechanisms of Aspergillus aculeatus-mediated abiotic stress tolerance in tallfescue[J]. Plant Physiology and Biochemistry, 2019, 142: 342-350.
[25] RusinowskiS, Szada-Borzyszkowska A, Zieleźnik-Rusinowska P, et al. How autochthonousmicroorganisms influence physiological status of Zea mays L. cultivated onheavy metal contaminated soils [J]. Environmental Science and PollutionResearch, 2019, 26(5): 4746-4763.
[26] Fusaro L,Salvatori E, Mereu S, et al. Photosynthetic traits as indicators forphenotyping urban and peri-urban forests: A case study in the metropolitan cityof Rome[J]. Ecological indicators, 2019, 103: 301-311.
[27] Pérez-RomeroJ A, Duarte B, Barcia-Piedras J M, et al. Investigating the physiologicalmechanisms underlying Salicornia ramosissima response to atmospheric CO2enrichment under coexistence of prolonged soil flooding and saline excess[J].Plant physiology and biochemistry, 2019, 135: 149-159.
[28] de SouzaLopes J, da Costa K C P, Fernandes V S, et al. Functional traits associated tophotosynthetic plasticity of young Brazil nut (Bertholletia excelsa) plants[J].Flora, 2019, 258: 151446.
[29] Bąba W,Kompała-Bąba A, Zabochnicka-Świątek M, et al. Discovering trends inphotosynthesis using modern analytical tools: More than 100 reasons to usechlorophyll fluorescence[J]. 2019.Photosynthetica 57(2):668-679
[30] Yang T Y,Cai L Y, Qi Y P, et al. BioMed Research International Increasing NutrientSolution pH Alleviated Aluminum-induced Inhibition of Growth and Impairment ofPhotosynthetic Electron Transport Chain in Citrus sinensis Seedlings[J]. BioMedResearch International,2019
[31] Çicek N,Kalaji H M, Ekmekci Y. Probing the photosynthetic efficiency of some Europeanand Anatolian Scots pine populations under UV-B radiation using polyphasicchlorophyll a fluorescence transient[J]. Photosynthetica 2020, 58(2):468-478
[32] Kalaji HM, Račková L, Paganová V, et al. Can chlorophyll-a fluorescence parameters beused as bio-indicators to distinguish between drought and salinity stress inTilia cordata Mill [J]. Environmental and Experimental Botany, 2018, 152:149-157.
[33] SamborskaI A, Kalaji H M, Sieczko L, et al. Structural and functional disorder in thephotosynthetic apparatus of radish plants under magnesium deficiency[J].Functional Plant Biology, 2018, 45(6): 668-679.
[34] Boguszewska‐Mańkowska D, Pieczyński M, Wyrzykowska A, et al. Divergent strategies displayed bypotato (Solanum tuberosum L.) cultivars to cope with soil drought[J]. Journalof agronomy and crop science, 2018, 204(1): 13-30.
[35] Kalaji HM, Bąba W, Gediga K, et al. Chlorophyll fluorescence as a tool for nutrientstatus identification in rapeseed plants[J]. Photosynthesis research, 2018, 136(3):329-343.
[36] Duarte B,Cabrita M T, Vidal T, et al. Phytoplankton community-level bio-opticalassessment in a naturally mercury contaminated Antarctic ecosystem (DeceptionIsland) [J]. Marine environmental research, 2018, 140: 412-421.
[37] Arslan Ö,Eyidoğan F, Ekmekçi Y. Freezing tolerance of chickpea: biochemical andmolecular changes at vegetative stage[J]. Biologia Plantarum, 2018, 62(1):140-148.
[38] DigradoA, Bachy A, Mozaffar A, et al. Long‐term measurements of chlorophyll a fluorescence using the JIP‐test show that combined abiotic stressesinfluence the photosynthetic performance of the perennial ryegrass (Loliumperenne) in a managed temperate grassland[J]. Physiologia plantarum, 2017,161(3): 355-371.
[39] MarcińskaI, Czyczyło-Mysza I, Skrzypek E, et al. Application of photochemical parametersand several indices based on phenotypical traits to assess intraspecificvariation of oat (Avena sativa L.) tolerance to drought[J]. Acta PhysiologiaePlantarum, 2017, 39(7): 153.
[40] DigradoA, Gourlez de la Motte L, Bachy A, et al. Long-term field study of theinfluence of the photosynthetic performance of temperate grassland species onecosystem CO2 exchange fluxes at the ecosystem-scale[J]. Gembloux Agro-biotech,2017.
[41] Yang X Q,Zhang Q S, Zhang D, et al. Light intensity dependent photosynthetic electrontransport in eelgrass (Zostera marina L.) [J]. Plant Physiology andBiochemistry, 2017, 113: 168-176.
[42] SemerciA, Semerci H, Çalişkan B, et al. Morphological and physiological responses todrought stress of European provenances of Scots pine[J]. European Journal ofForest Research, 2017, 136(1): 91-104.
[43] MajewskaM L, Rola K, Zubek S. The growth and phosphorus acquisition of invasive plantsRudbeckia laciniata and Solidago gigantea are enhanced by arbuscularmycorrhizal fungi[J]. Mycorrhiza, 2017, 27(2): 83-94.
[44] Kalaji HM, Schansker G, Brestic M, et al. Frequently asked questions about chlorophyllfluorescence, the sequel[J]. Photosynthesis Research, 2017, 132(1): 13-66.
[45] DąbrowskiP, Baczewska A H, Pawluśkiewicz B, et al. Prompt chlorophyll a fluorescence asa rapid tool for diagnostic changes in PSII structure inhibited by salt stressin Perennial ryegrass[J]. Journal of Photochemistry and Photobiology B:Biology, 2016, 157: 22-31.
[46] Kalaji HM, Jajoo A, Oukarroum A, et al. Chlorophyll a fluorescence as a tool to monitorphysiological status of plants under abiotic stress conditions[J]. Actaphysiologiae plantarum, 2016, 38(4): 102.
[47] PollastriniM, Holland V, Brüggemann W, et al. Taxonomic and ecological relevance of thechlorophyll a fluorescence signature of tree species in mixed Europeanforests[J]. New Phytologist, 2016, 212(1): 51-65.
[48] Canton GC, Bertolazi A A, Cogo A J D, et al. Biochemical and ecophysiological responsesto manganese stress by ectomycorrhizal fungus Pisolithus tinctorius and inassociation with Eucalyptus grandis[J]. Mycorrhiza, 2016, 26(5): 475-487.
[49] JurczykB, Rapacz M, Pociecha E, et al. Changes in carbohydrates triggered by lowtemperature waterlogging modify photosynthetic acclimation to cold in Festucapratensis[J]. Environmental and experimental botany, 2016, 122: 60-67.
[50] Bąba W,Kalaji H M, Kompała-Bąba A, et al. Acclimatization of photosynthetic apparatusof tor grass (Brachypodium pinnatum) during expansion[J]. PloS one, 2016,11(6).
[51] Ogar A,Sobczyk Ł, Turnau K. Effect of combined microbes on plant tolerance to Zn–Pbcontaminations[J]. Environmental Science and Pollution Research, 2015, 22(23):19142-19156.
[52] Østrem L,Rapacz M, Larsen A, et al. Influences of growth cessation and photoacclimationon winter survival of non-native Lolium–Festuca grasses in high-latituderegions[J]. Environmental and Experimental Botany, 2015, 111: 21-31.
[53] Fusaro L,Salvatori E, Mereu S, et al. Urban and peri-urban forests in the metropolitanarea of Rome: Ecophysiological response of Quercus ilex L. in two greeninfrastructures in an ecosystem services perspective[J]. Urban Forestry &Urban Greening, 2015, 14(4): 1147-1156.
[54] Tyrka M,Rapacz M, Fiust A, et al. Quantitative trait loci mapping of freezing toleranceand photosynthetic acclimation to cold in winter two‐and six‐rowed barley[J]. Plant Breeding, 2015, 134(3): 271-282.
[55] Kalaji HM, Oukarroum A, Alexandrov V, et al. Identification of nutrient deficiency inmaize and tomato plants by in vivo chlorophyll a fluorescence measurements[J].Plant physiology and biochemistry, 2014, 81: 16-25.
[56] GiovenzanaV, Beghi R, Buratti S, et al. Monitoring of fresh-cut Valerianella locustaLaterr. shelf life by electronic nose and VIS–NIR spectroscopy[J]. Talanta,2014, 120: 368-375.
[57] Żurek G,Rybka K, Pogrzeba M, et al. Chlorophyll a fluorescence in evaluation of theeffect of heavy metal soil contamination on perennial grasses[J]. Plos one,2014, 9(3).
[58] SalvatoriE, Fusaro L, Gottardini E, et al. Plant stress analysis: Application of prompt,delayed chlorophyll fluorescence and 820 nm modulated reflectance. Insightsfrom independent experiments[J]. Plant physiology and biochemistry, 2014, 85:105-113.
[59] Zurek G,Rybka K, Pogrzeba M, et al. Chlorophyll a Fluorescence in Evaluation of theEffect of Heavy Metal Soil Contamination[J]. Plos one,2014.
[60] BlumenthalJ, Megherbi D B, Lussier R. Unsupervised machine learning via Hidden MarkovModels for accurate clustering of plant stress levels based on imagedchlorophyll fluorescence profiles & their rate of change in time[C]//2014IEEE International Conference on Computational Intelligence and VirtualEnvironments for Measurement Systems and Applications (CIVEMSA). IEEE, 2014:76-81.
[61] VanHeerden P D R. Differential acclimation capacity to frost in sugarcanevarieties grown under field conditions[J]. Plant Growth Regulation, 2014,72(2): 181-187.
[62] PotgieterC, De Beer M, Claassens S. The effect of canola (Brassica napus) as abiofumigant on soil microbial communities and plant vitality: a pot study[J].South African Journal of Plant and Soil, 2013, 30(4): 191-201.
[63] BresticM, Zivcak M. PSII fluorescence techniques for measurement of drought and hightemperature stress signal in crop plants: protocols andapplications[M]//Molecular stress physiology of plants. Springer, India, 2013:87-131.
[64] GoltsevV, Zaharieva I, Chernev P, et al. Drought-induced modifications ofphotosynthetic electron transport in intact leaves: analysis and use of neuralnetworks as a tool for a rapid non-invasive estimation[J]. Biochimica etBiophysica Acta (BBA)-Bioenergetics, 2012, 1817(8): 1490-1498.
[65] Jones MO, Piron-Prunier F, Marcel F, et al. Characterisation of alleles of tomatolight signalling genes generated by TILLING[J]. Phytochemistry, 2012, 79:78-86.
[66] GoltsevV, Zaharieva I, Chernev P, et al. Drought-induced modifications ofphotosynthetic electron transport in intact leaves: analysis and use of neuralnetworks as a tool for a rapid non-invasive estimation[J]. Biochimica etBiophysica Acta (BBA)-Bioenergetics, 2012, 1817(8): 1490-1498.
[67] MaboetaM, van Rensburg L. Vermicomposting of Industrial Organic Wastes and itsApplication in Mine Rehabilitation Strategies–An Overview from a South AfricanPerspective[J].2012
[68] Wagg S,Mills G, Hayes F, et al. OZONE AND DROUGHT STRESS INTERACTIONS IN BEAN ANDGRASSLAND SPECIES[J]. Programme &, 2011: 33.
[69] PollastriniM, Desotgiu R, Cascio C, et al. Growth and physiological responses to ozone andmild drought stress of tree species with different ecological requirements[J].Trees, 2010, 24(4): 695-704.
[70] Torres-RomeroD, King-Diaz B, Strasser R J, et al. Friedelane triterpenes from Celastrusvulcanicola as photosynthetic inhibitors[J]. Journal of agricultural and foodchemistry, 2010, 58(20): 10847-10854.
[71] Kirova M,Ceppi G, Chernev P, et al. Using artificial neural networks for plant taxonomicdetermination based on chlorophyll fluorescence induction curves[J].Biotechnology & Biotechnological Equipment, 2009, 23(sup1): 941-945.
[72] Pérez-MéndezA, Maldonado-Rodríguez R, Torres-Rivas E, et al. Pisum sativum classificationbased on a methodological approach for pattern recognition using discriminantanalysis and neural networks[J]. stress, 2005, 8(17): 18.
[73] Soja G,Soja A. Recognizing the sources of stress in wheat and bean by usingchlorophyll fluorescence induction parameters as inputs for neural networkmodels[J]. PHYTON-HORN-, 2005, 45(3): 157.